LEARN ABOUT
Pharmacovigilance
Mandate
Department of Pharmacovigilance and Clinical Trials at BoMRA is responsible for the following functions:
- Pharmacovigilance - Safety monitoring of human medicines, vaccines, veterinary medicines, and medical devices.
- Oversight and Regulation of Clinical Trials
- Post Marketing Surveillance of medical products for quality defects including Substandard and Falsified Medicines.
Organogram
 CEOHead of Department
CEOHead of Department MainSupervisor
MainSupervisor MidStaff
MidStaff MidStaff
MidStaff MidStaff
MidStaff
 MainSupervisor
MainSupervisor MidStaff
MidStaff MidStaff
MidStaff MidStaff
MidStaff
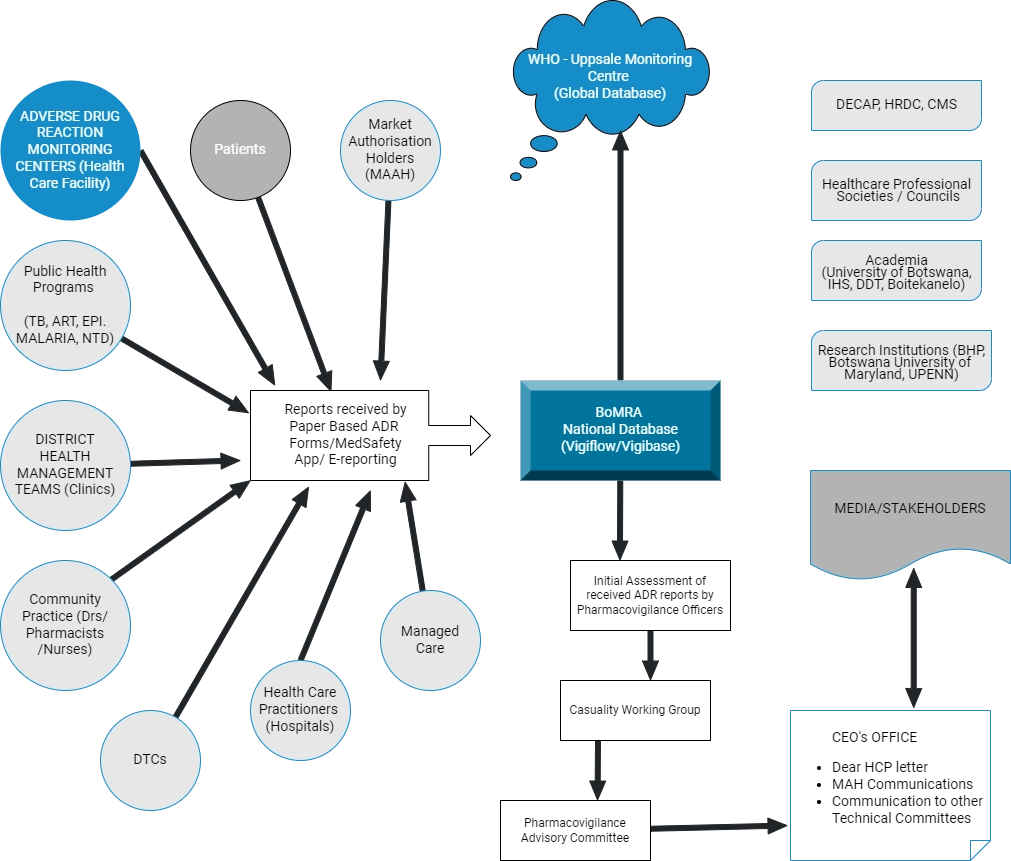
To achieve its mandate and objective the Directorate/Department works very closely with different stakeholders including;
- Public Health Programmes
- District Health Management Teams
- Professional bodies
- Academia
- Healthcare professionals
- Healthcare Facilities
- Market Authorisation Holders
- Research Institutions
- Patients
Below is a graphical presentation of the stakeholders and how each fits into the medicines/vaccine’s safety surveillance activities.